SOLVOMET/SIM² KU Leuven researchers developed a novel solvometallurgical approach to recover cobalt from cathode materials of lithium-ion batteries (LIBs). The approach uses a choline chloride citric acid based deep eutectic solvent (DES) to leach the cathode material in presence of metallic aluminium and copper, which are current collectors that are commonly present in LIBs. Hereafter, the pregnant DES leachate is used as an alternative more polar in non-aqueous solvents extraction (non-aq SX) processes, resulting in the successful separation and recovery of cobalt as a cobalt(II) oxalate precipitate. This work was supported by the EU HORIZON 2020 CROCODILE project and published in the journal Green Chemistry.
Secondary cobalt resources
Cobalt is commonly used in the cathode material of lithium-ion batteries. These batteries are increasingly applied in devices such as smart phones and electrical vehicles, due to their excellent capacity compared with other batteries. Since the use of electrical vehicles is strongly promoted because of environmentally issues, Europe can risk shortcomings of cobalt in the future. Therefore, recycling of these spent lithium-ion batteries becomes important.
Hydrometallurgical secondary recovery routes usually first treat the crushed spent batteries with mineral acids, followed by the separation and recovery of the metals via techniques such as precipitation or solvent extraction.
Deep-Eutectic Solvents
However, a novel approach is to treat the spent batteries with deep‑eutectic solvents instead of mineral acids. This ensures a significant environmental benefit because it avoids the emission of toxic gasses, like for example chlorine gas that is produced during treatment with a commonly used acid such as hydrochloric acid.
Furthermore, after treatment the deep‑eutectic solvent can be used for the metal separation and recovery as well. Hereby a novel branch of solvent extraction is applied, namely non‑aqueous solvent extraction. The latter approach also yields economic and environmental benefits, as in this work where the addition of alkali or acids is avoided during the extraction processes.
LiCoO2
This work treated a common lithium-ion battery cathode material (LiCoO2) in the presence of its current collectors (Al and Cu) with a choline chloride‑citric acid based deep‑eutectic solvent, recovering 81% of cobalt in the form of cobalt oxalate with a purity of 99.9%. A schematic overview of this work is shown in Figure 1.

Figure 1: Summary of the solvometallurgical recovery of cobalt from LiCoO2 using DESs.
Solvometallurgical recovery process
Solvoleaching. LiCoO2 powder was leached by a choline chloride‑citric acid DES diluted with 35 wt% of water. Metallic aluminium and copper powder were added at their average weight composition in LIBs. Cobalt was quantitatively leached by the DES after one hour at 40 °C and assisted by the presence of copper.
Mechanistic studies confirmed the leaching of cobalt from LiCoO2 is driven by three factors. Firstly, metallic copper reduces cobalt from its trivalent state to its more soluble divalent state. Thus, copper was found to be the decisive reducing agent in the system, while aluminium was ineffective because it was more prone to side reactions.
Secondly, acidic protons from the DES attack the LiCoO2 crystal structure and partially open it, causing efficient leaching of lithium and cobalt.
Thirdly, the chloride anions in the DES stabilise the divalent cobalt cations in the pregnant leachate, with tetrachlorocobaltate being the predominant species. A summary of this leaching process is shown in Figure 2.
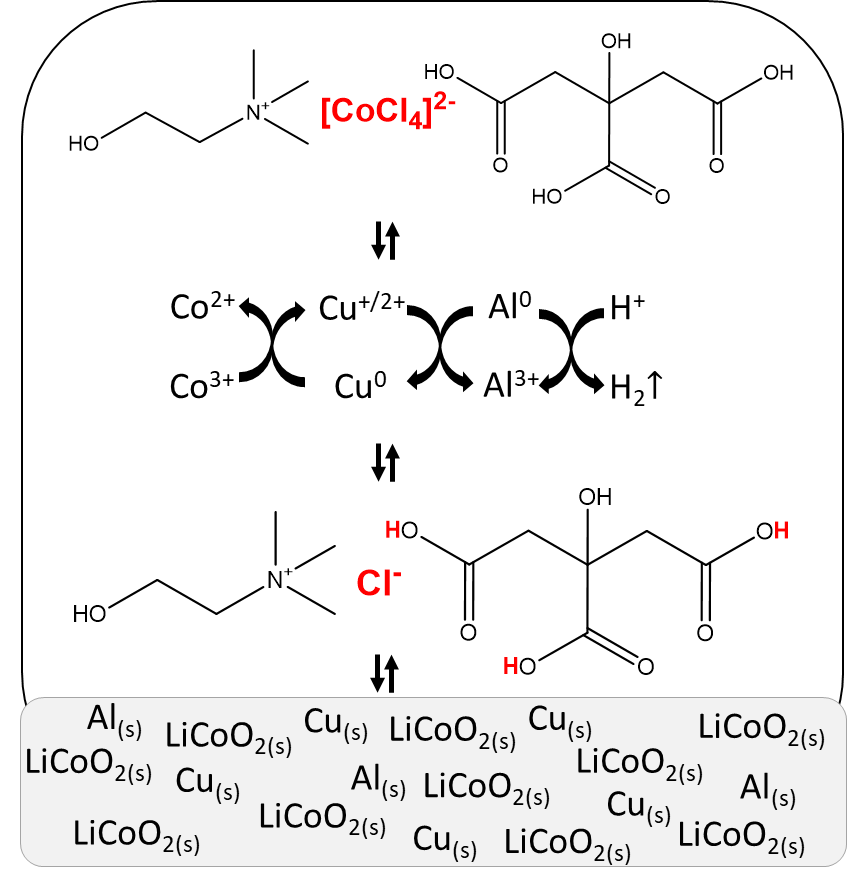
Figure 2: Summary of the solvoleaching process.
Non‑aqueous solvent extraction. After leaching, a pregnant DES leachate was obtained which contained cobalt, copper, lithium and aluminium. Cobalt was then separated and recovered by using non‑aqueous solvent extraction, whereby the conventional aqueous polar phase is replaced by the loaded DES phase.
Firstly copper was quantitatively extracted by the commercial extractant LIX 984 (diluted 60 vol% in an aliphatic diluent). After the removal of copper, cobalt was quantitatively and selectively extracted over aluminium and lithium by the commercial ionic liquid Aliquat 336 (pre-saturated by the DES).
Both copper and cobalt were quantitatively stripped form their corresponding loaded organic phases by 0.25 mol L-1 oxalic acid, producing oxalate precipitates.
Cobalt oxalate is easily converted to cobalt oxide by calcination, which is a commonly used precursor for the production of LIBs. A summary of the non-aqueous solvent extraction processes is shown in Figure 3.
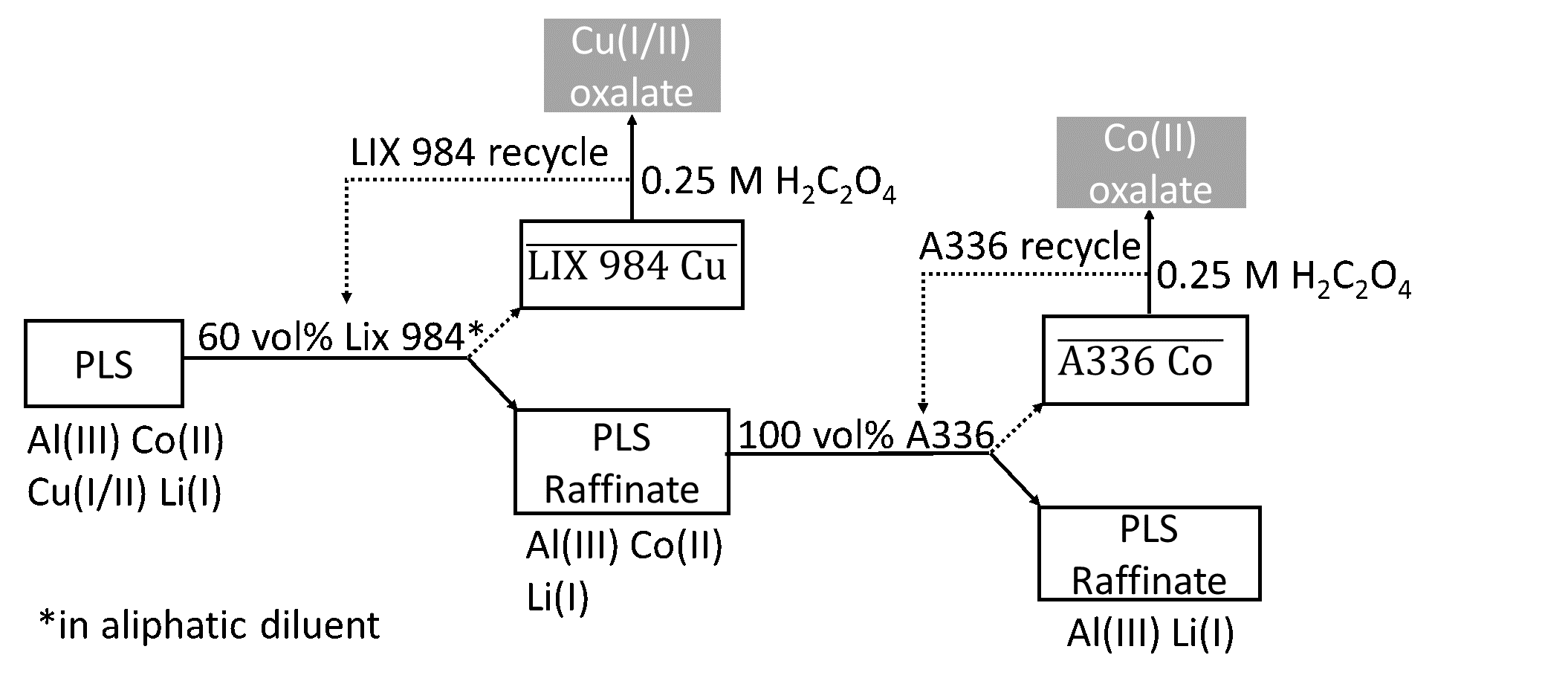
Figure 3: Schematic overview of the applied non-aqueous solvent extraction processes.
Full reference article
Peeters, N., Binnemans, K., & Riaño, S. (2020). Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chemistry, https://doi.org/10.1039/D0GC00940G
Biography Nand Peeters
 Nand Peeters is a PhD researcher working with Prof. Dr. Koen Binnemans (SOLVOMET team) at KU Leuven. He obtained his master degree in chemistry in June 2018 and started his PhD in August that same year. His PhD is funded by the H2020 CROCODILE project. His main research interests are focused on hydrometallurgy, with leaching and solvent extraction being his main work of his PhD.
Nand Peeters is a PhD researcher working with Prof. Dr. Koen Binnemans (SOLVOMET team) at KU Leuven. He obtained his master degree in chemistry in June 2018 and started his PhD in August that same year. His PhD is funded by the H2020 CROCODILE project. His main research interests are focused on hydrometallurgy, with leaching and solvent extraction being his main work of his PhD.
Acknowledgements
This research project has received funding from the European Union's EU Framework Programme for Research and Innovation Horizon 2020 under Grant Agreement No 776473 (CROCODILE). This work reflects only the author's view, exempting the Community from any liability. Website: https://h2020-crocodile.eu/.

